Page 36 - ICC Science Dictionary (spreads)
P. 36
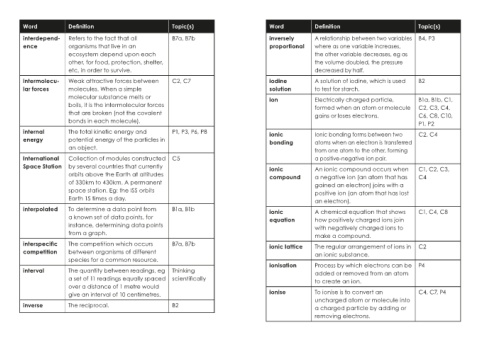
Word Definition Topic(s) Word Definition Topic(s)
interdepend- Refers to the fact that all B7a, B7b inversely A relationship between two variables B4, P3
ence organisms that live in an proportional where as one variable increases,
ecosystem depend upon each the other variable decreases, eg as
other, for food, protection, shelter, the volume doubled, the pressure
etc, in order to survive. decreased by half.
intermolecu- Weak attractive forces between C2, C7 iodine A solution of iodine, which is used B2
lar forces molecules. When a simple solution to test for starch.
molecular substance melts or ion Electrically charged particle, B1a, B1b, C1,
boils, it is the intermolecular forces formed when an atom or molecule C2, C3, C4,
that are broken (not the covalent gains or loses electrons. C6, C8, C10,
bonds in each molecule). P1, P2
internal The total kinetic energy and P1, P3, P6, P8 ionic Ionic bonding forms between two C2, C4
energy potential energy of the particles in bonding atoms when an electron is transferred
an object. from one atom to the other, forming
International Collection of modules constructed C5 a positive-negative ion pair.
Space Station by several countries that currently ionic An ionic compound occurs when C1, C2, C3,
orbits above the Earth at altitudes compound a negative ion (an atom that has C4
of 330km to 430km. A permanent gained an electron) joins with a
space station. Eg: the ISS orbits positive ion (an atom that has lost
Earth 15 times a day. an electron).
interpolated To determine a data point from B1a, B1b ionic A chemical equation that shows C1, C4, C8
a known set of data points, for equation how positively charged ions join
instance, determining data points with negatively charged ions to
from a graph. make a compound.
interspecific The competition which occurs B7a, B7b ionic lattice The regular arrangement of ions in C2
competition between organisms of different an ionic substance.
species for a common resource.
ionisation Process by which electrons can be P4
interval The quantity between readings, eg Thinking added or removed from an atom
a set of 11 readings equally spaced scientifically to create an ion.
over a distance of 1 metre would
give an interval of 10 centimetres. ionise To ionise is to convert an C4, C7, P4
uncharged atom or molecule into
inverse The reciprocal. B2 a charged particle by adding or
removing electrons.